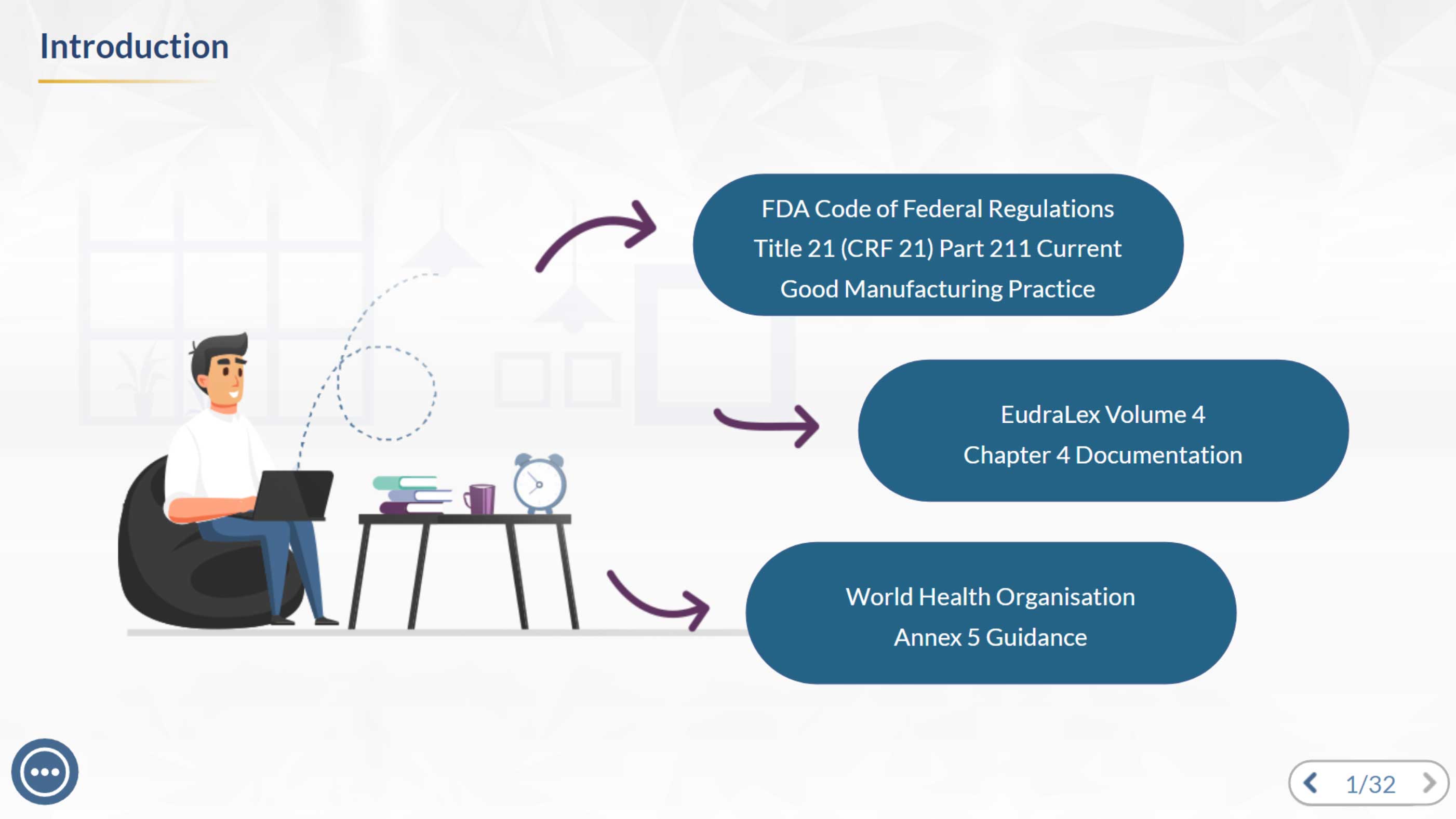
Learn the essentials of GMP document control—how documents are created, maintained, and archived to ensure data integrity and compliance. This module provides quick, practical guidance for stronger quality and audit readiness.
This interactive module, designed in Articulate Storyline using modern instructional design strategies, guides learners through the complete lifecycle of GMP documents—from creation and maintenance to secure archiving. Built for clarity and compliance, it helps teams strengthen data integrity, improve document control practices, and stay audit-ready in a regulated GMP environment.