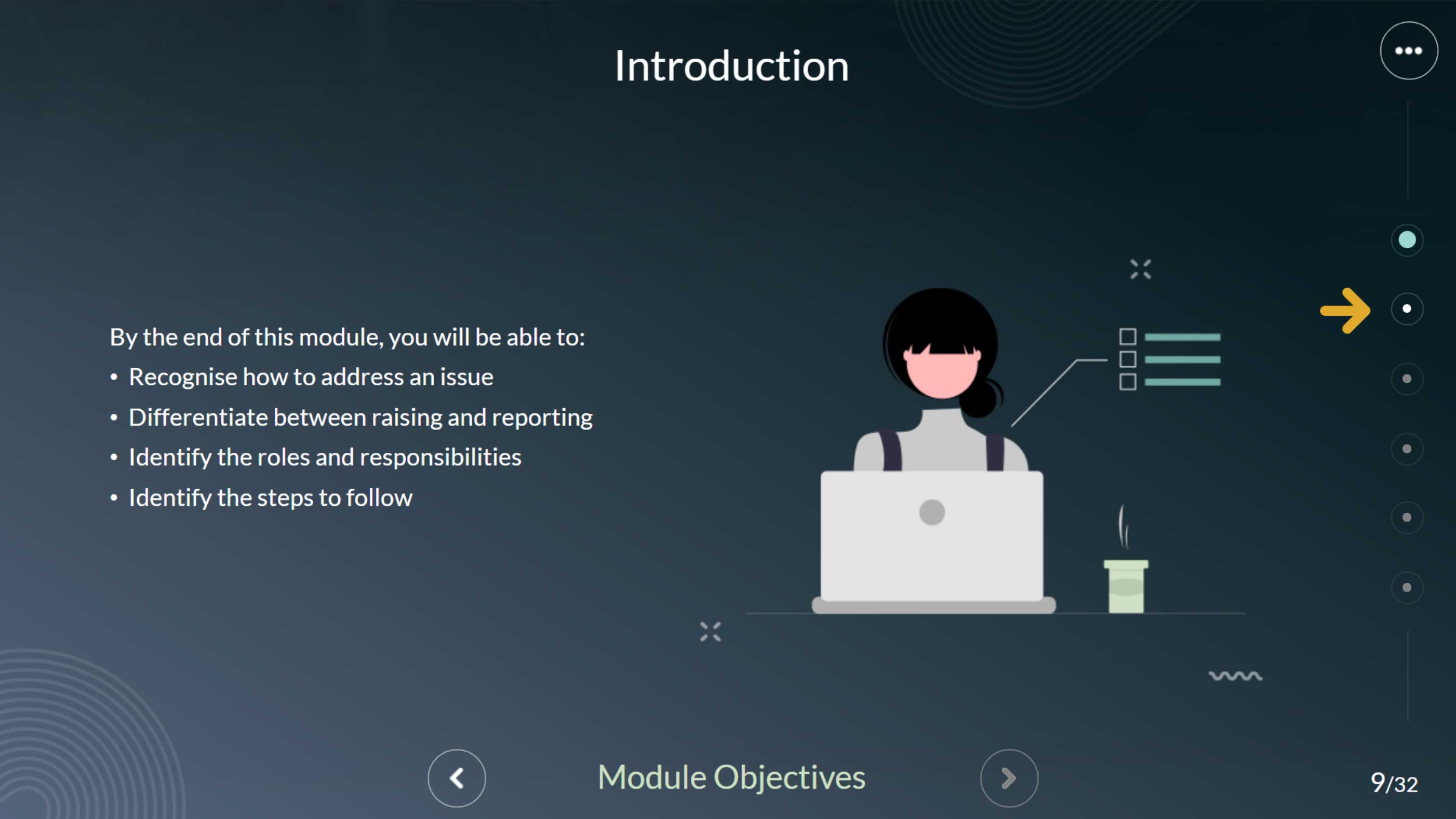
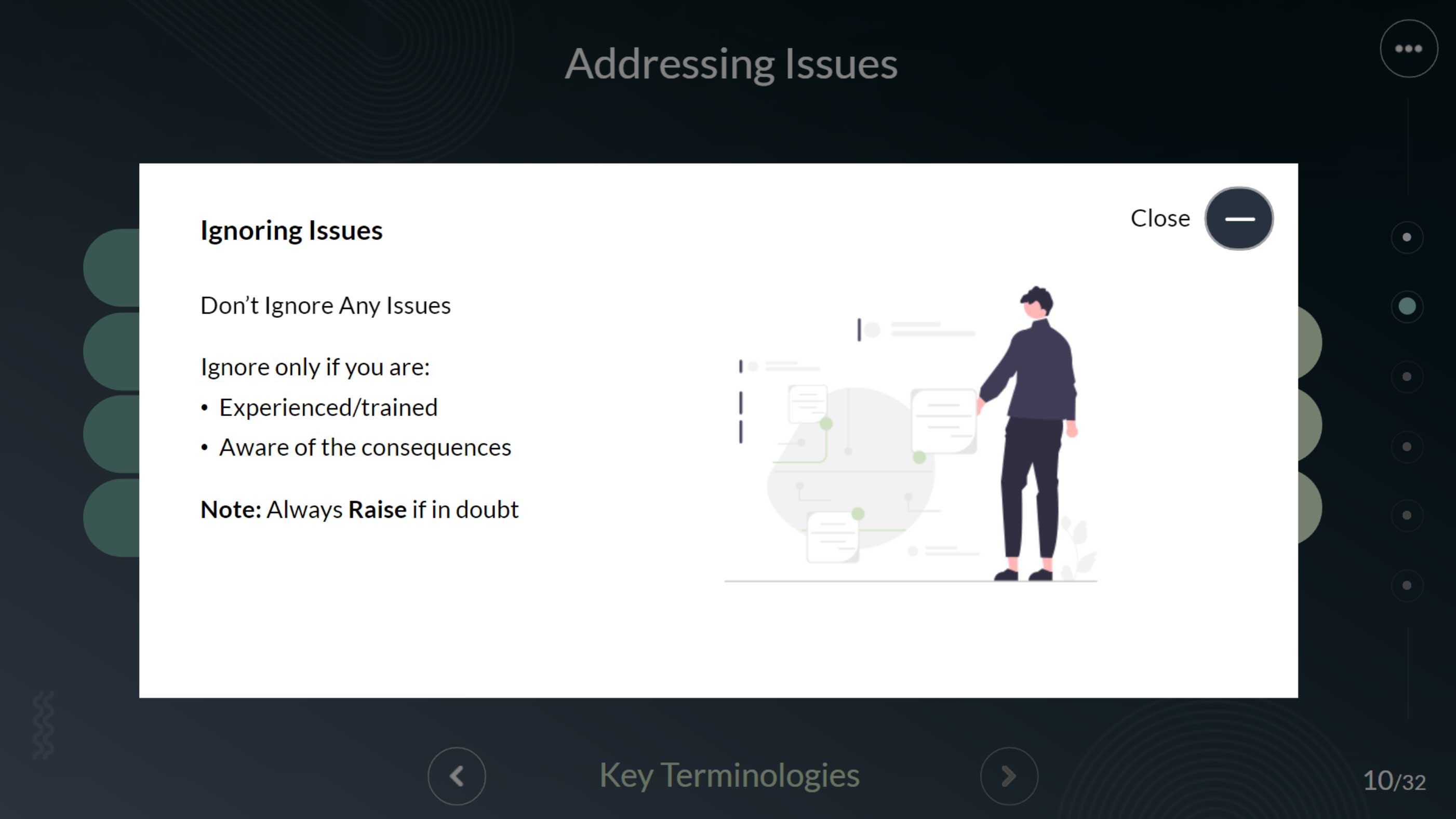
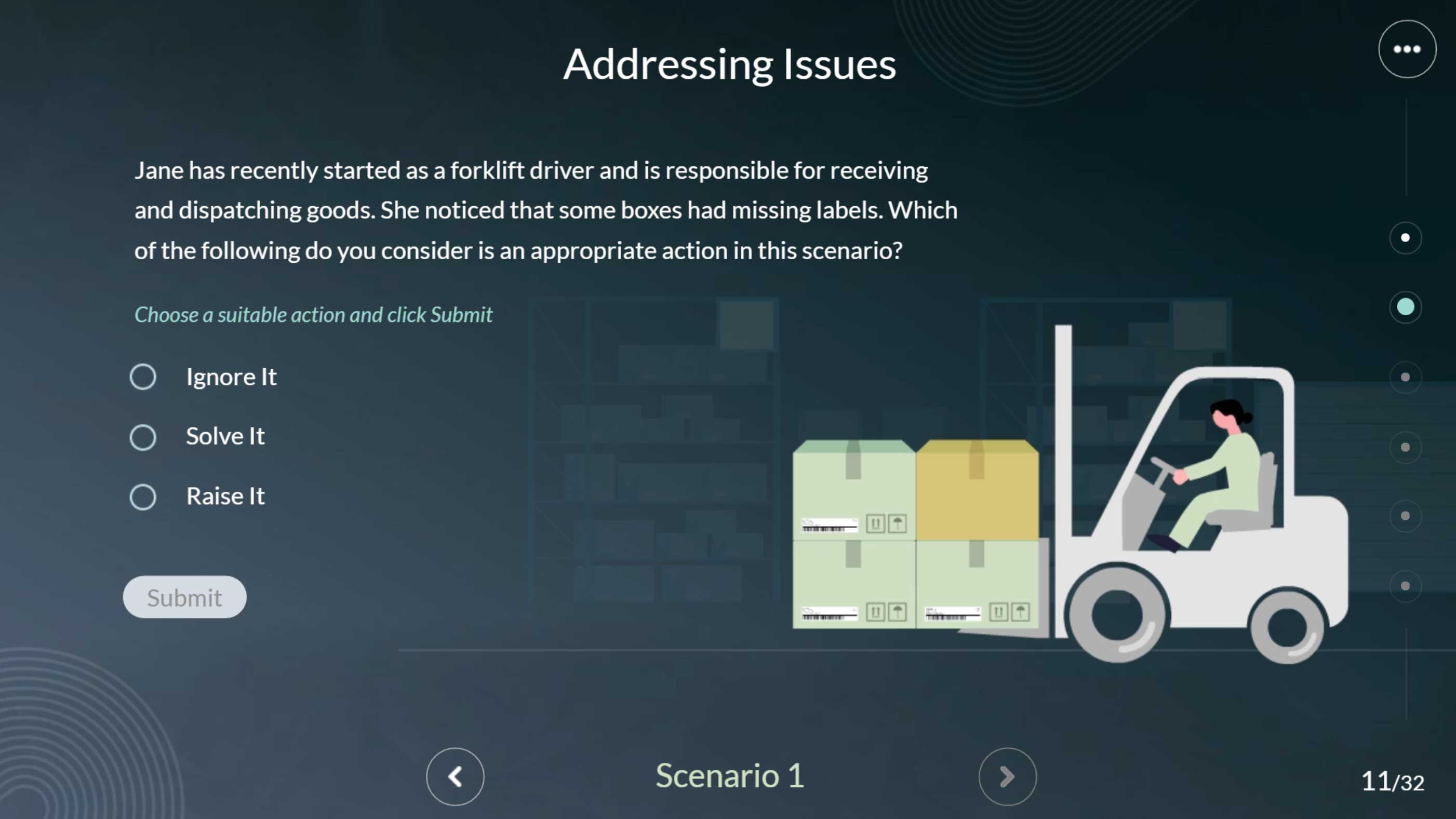
This module provides a clear, structured overview of how to manage quality issues in a GMP environment—explaining the who, when, why, and how behind a systematic and efficient issue-handling process.
This interactive Articulate Storyline module uses proven instructional design techniques to help GMP personnel confidently identify, report, and manage day-to-day quality events. In pharmaceutical environments, unexpected issues are inevitable—so understanding the who, when, why, and how of quality event reporting is essential for maintaining compliance and product integrity. Through clear, practical scenarios, this module explains how to raise, record, and resolve quality issues systematically and efficiently, empowering teams to respond quickly and uphold GMP standards.